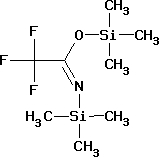
बीआईएस (ट्राइमेथिलसिल) ट्राइफ्लुओरोएसिटामाइड
उत्पाद विवरण:
- आण्विक सूत्र C8H18F3NOSi2
- फ़्लैश पॉइंट 12°C (closed cup)
- शेल्फ लाइफ 12 months
- घनत्व ग्राम प्रति घन सेंटीमीटर (g/cm3)
- साइज Customizable (commonly 5 mL, 25 mL, 100 mL)
- पैकेजिंग का प्रकार Sealed bottle
- भौतिक अवस्था Liquid
- अधिक देखने के लिए क्लिक करें
बीआईएस (ट्राइमेथिलसिल) ट्राइफ्लुओरोएसिटामाइड मूल्य और मात्रा
- 500
- मिलीमीटर/मिलीमीटर
बीआईएस (ट्राइमेथिलसिल) ट्राइफ्लुओरोएसिटामाइड उत्पाद की विशेषताएं
- Customizable (commonly 5 mL, 25 mL, 100 mL)
- Clear colorless to pale yellow liquid
- 12 months
- ग्राम प्रति घन सेंटीमीटर (g/cm3)
- 12°C (closed cup)
- C8H18F3NOSi2
- ≥99.0
- Derivatization reagent in gas chromatography
- ≥99.0%
- 259.36 g/mol
- Liquid
- Sealed bottle
- <0°C
- Not determined
- Store in a cool, dry place; keep container tightly closed
उत्पाद वर्णन
Bis (Trimethylsilyl) Trifluoroacetamide
Commenced in the year 1962, we are recognized in the market as the most dependable manufacturer and exporter of Bis (trimethylsilyl) trifluoroacetamide. It is used as derivatives for hydroxyl and other stable trimethylsilyl group chemicals. To process this chemical, our diligent workforce uses quality grade compounds, sourced from trusted vendors. Bis (trimethylsilyl) trifluoroacetamide is also used for analytical purposes and as a reagent for chemical synthesis. We offer this chemical at market leading prices to clients.
Features:
-
Colorless and odorless
-
Low melting point
-
Excellent silylation reagent
Further Details:
LC583 Bis (trimethylsilyl) trifluoroacetamide
| Order number | Packaging | Quantity | Price |
| AC27583 | Glass bottle | 5 ml | 35.325 |
| AC27583 | Glass bottle | 25 ml | 140.40 |
| Product information | |
| Synonyms | 2,2,2-Trifluoro-N,O-bis(trimethylsilyl)acetamide |
| Hill Formula | C8H18F3NOSi2 |
| HS Code | 2931 00 99 |
| EC number | 247-103-9 |
| Molar mass | 257.40 g/mol |
| CAS number | 25561-30-2 |
| Chemical and physical data | |
| Solubility | (20oC) Hydrolysis |
| Molar mass | 257.40 g/mol |
| Density | 0.97 g/cm3 (20oC) |
| Boiling point | 145oC |
| Flash point | 34oC |
| Safety information according to GHS | |
| Hazard Statement(s) | H226: Flammable liquid and vapour. |
| Precautionary Statement(s) | P210: Keep away from heat. |
| Signal Word | Warning |
| Hazard Pictogram(s) | |
| Storage class | 3 Flammable Liquids |
| WGK | WGK 2 water endangering |
| Disposal | 3 Relatively unreactive organic reagents should be collected in container A. If halogenated, they should be collected in container B. For solid residues use container C. |
| Safety information | |
| R Phrase | R 10 Flammable. |
| S Phrase | S 23-26 Do not breathe vapour.In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| श्रेणियाँ of danger | flammable, irritant |
| Hazard Symbol | Irritant |
| R Phrase | R 10 Flammable. |
| श्रेणियाँ of danger | flammable |
| Transport information | |
| Declaration (transport by sea) IMDG-Code | UN 1993 FLAMMABLE LIQUID, N.O.S.(BIS(TRIMETHYLSILYL)-TRIFLUOROACETAMIDE), 3, III |
| Declaration (transport by air) IATA-DGR | UN 1993 FLAMMABLE LIQUID, N.O.S.(BIS(TRIMETHYLSILYL)-TRIFLUOROACETAMIDE), 3, III |
| Specifications | |
| Assay (GC, area%) | 98 % |
| Identity (IR) | passes test |
Versatile Silylation Reagent for Analytical Chemistry
BSTFA stands as a premier choice for analytical laboratories involved in gas chromatography. Its remarkable efficiency in converting polar compounds enhances volatility and detector response, making it invaluable for diverse sample matrices. With its high purity and reliable performance, BSTFA improves accuracy and reproducibility in qualitative and quantitative analyses.
Proper Handling and Storage
Due to its high reactivity and sensitivity to moisture, it is crucial to handle BSTFA with care. Always employ gloves, safety goggles, and use a fume hood during operations. Seal the container tightly after use and store in a cool, dry environment to maintain product stability and extend shelf life.
FAQs of Bis (Trimethylsilyl) Trifluoroacetamide:
Q: How should Bis (Trimethylsilyl) Trifluoroacetamide be used in sample preparation?
A: BSTFA is primarily used as a silylation reagent for derivatization in gas chromatography. Add it to your sample dissolved in an appropriate organic solvent, and allow the reagent to react with target functional groups. For best results, perform the process in a moisture-free environment to prevent hydrolysis.Q: What protective measures are required when handling BSTFA?
A: Since BSTFA is highly flammable and can cause irritation, you should always wear gloves and safety goggles. Additionally, manipulation of the reagent should be done within a properly functioning fume hood to minimize inhalation exposure.Q: When should BSTFA be used in analytical workflows?
A: BSTFA is ideal for use during the sample preparation stage in gas chromatography, especially when analytes need to be derivatized to improve volatility or detectability. Apply it immediately before analysis to ensure maximum efficacy.Q: Where should BSTFA be stored to ensure its stability?
A: Store BSTFA in a tightly sealed, original bottle placed in a cool, dry location. Exposure to moisture can lead to hydrolysis, thus reducing reagent effectiveness, so minimize container openings and tightly close after each use.Q: What are the main benefits of using BSTFA in gas chromatography?
A: BSTFA efficiently derivatizes polar functional groups, improving analyte volatility and enhancing chromatographic behavior. This leads to sharper peaks, better quantification, and increased sensitivity in analytical results.Q: How long is the shelf life of BSTFA, and how can it be maximized?
A: BSTFA typically has a shelf life of 12 months when stored properly. To maximize its lifespan, ensure the bottle is tightly closed after each use and kept away from moisture and high temperatures.Q: What is the process for safely disposing of BSTFA after use?
A: Dispose of BSTFA as hazardous laboratory waste according to institutional and local regulations. Do not pour unused reagent down the drain. Use appropriate waste containers and coordinate with your safety officer for disposal.
Price: Â
- 50
- 100
- 200
- 250
- 500
- 1000+
Laboratory Chemicals अन्य उत्पाद
“केवल 500ml तक के ऑर्डर स्वीकार करने वाले रिटेल सौदे”।
 |
ALPHA CHEMIKA
सर्वाधिकार सुरक्षित.(उपयोग की शर्तें) इन्फोकॉम नेटवर्क प्राइवेट लिमिटेड . द्वारा विकसित एवं प्रबंधित |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese