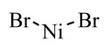निकेल (Ii) ब्रोमाइड
उत्पाद विवरण:
- स्टोरेज निर्देश Dry Place
- उपयोग Lab Chemicals
- ग्रेड Industrial
- एप्लीकेशन Lab Chemicals
- शुद्धता (%) 99.99
- अधिक देखने के लिए क्लिक करें
X
निकेल (Ii) ब्रोमाइड मूल्य और मात्रा
- 500
- मिलीलीटर/मिलिलिटर
निकेल (Ii) ब्रोमाइड उत्पाद की विशेषताएं
- Lab Chemicals
- Lab Chemicals
- Industrial
- 99.99
- Dry Place
उत्पाद वर्णन
Nickel (Ii) BromideLC4245 Nickel(II) bromide for synthesis Order number Packaging Quantity Price AC31245 Glass bottle 50 g 33.44 Product information Synonyms Nickel dibromide Hill Formula Br2Ni Chemical formula NiBr2 HS Code 2827 59 00 EC number 236-665-0 Molar mass 218.5g/mol EC index number 028-029-00-4 CAS number 13462-88-9 Chemical and physical data Solubility (20 C) (reaction) Melting point 1460 C Molar mass 218.5g/mol Density 5.09g/cm3 Safety information according to GHS Hazard Statement(s) H350i: May cause cancer by inhalation.H341: Suspected of causing genetic defects.H360D: May damage the unborn child.H372: Causes damage to organs through prolonged or repeated exposure.H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled.H317: May cause an allergic skin reaction.H410: Very toxic to aquatic life with long lasting effects. Precautionary Statement(s) P201: Obtain special instructions before use.P273: Avoid release to the environment.P280: Wear protective gloves.P281: Use personal protective equipment as required.P301 + P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician.P302 + P352: IF ON SKIN: Wash with plenty of soap and water.P304 + P341: IF INHALED: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing.P308 + P313: IF exposed or concerned: Get medical advice/attention.P342 + P311: If experiencing respiratory symptoms: Call a POISON CENTER or doctor/physician. Signal Word Danger Hazard Pictogram(s) Storage class Non combustible substances, toxic WGK WGK 3 highly water endangering Disposal 15Heavy metal-containing solutions and solids: container E. Stir Raney nickel (also: Urushibara nickel) in the form of an aqueous suspension into hydrochloric acid (Cat. No. 100312) until dissolved (container E). Neither Raney nickel itself nor any filter residues should be allowed to dry out, otherwise they will spontaneously ignite in air. In this context, heavy metal means any compound of antimony, arsenic, cadmium, chromium(VI), copper, lead, nickel and tin, as well as these subtances in metallic form, if they are classified as hazardous (acc. to AbfallverzeichnisV - Waste Catalogue Ordinance, Appendix 3). Other heavy metals should be collected separately. Safety information R Phrase R 49-61-42/43-48/23-68-50/53May cause cancer by inhalation.May cause harm to the unborn child.May cause sensitization by inhalation and skin contact.Also toxic: danger of serious damage to health by prolonged exposure through inhalation.Possible risk of irreversible effects.Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. S Phrase S 53-45-60-61Avoid exposure - obtain special instructions before use.In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible).This material and its container must be disposed of as hazardous waste.Avoid release to the environment. Refer to special instructions/ Safety data sheets. श्रेणियाँofdanger carcinogenic, toxic for reproduction, sensitizing, toxic, mutagenic, dangerous for the environment Hazard Symbol Toxic Dangerous for the environment Transport information Declaration (transport by sea) IMDG-Code UN 3077 ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.(NICKEL(II)-BROMIDE), 9, III, Marine Pollutant: P Declaration (transport by air) IATA-DGR UN 3077 ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.(NICKEL(II)-BROMIDE), 9, III Specifications Assay of Ni 98%FAQs of Nickel (Ii) Bromide:
Q: What is the purity of Nickel (II) Bromide?
A: The purity of Nickel (II) Bromide is 99.99%.Q: What is the recommended storage condition for Nickel (II) Bromide?
A: Nickel (II) Bromide should be stored in a dry place.Q: What grade is Nickel (II) Bromide classified under?
A: Nickel (II) Bromide is classified under the Industrial grade.Q: What is the primary application of Nickel (II) Bromide?
A: Nickel (II) Bromide is primarily used as a lab chemical.Q: Can Nickel (II) Bromide be used in laboratory settings?
A: Yes, Nickel (II) Bromide is specifically designed for laboratory applications.Tell us about your requirement

Price: Â
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
मोबाइल number
Email
Laboratory Chemicals अन्य उत्पाद
हम लैब केमिकल्स में सौदे कर रहे हैं।
“केवल 500ml तक के ऑर्डर स्वीकार करने वाले रिटेल सौदे”।
“केवल 500ml तक के ऑर्डर स्वीकार करने वाले रिटेल सौदे”।
 |
ALPHA CHEMIKA
सर्वाधिकार सुरक्षित.(उपयोग की शर्तें) इन्फोकॉम नेटवर्क प्राइवेट लिमिटेड . द्वारा विकसित एवं प्रबंधित |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese