Boron hydride dimethylamine
Boron hydride dimethylamine Specification
- Usage
- Lab Chemicals
- Storage Instructions
- Dry Place
- Grade
- Industrial
- Type
- Industrial Lab Chemicals
- Application
- Lab Chemicals
- Purity(%)
- 99.99
About Boron hydride dimethylamine
LC1119 Boron hydride dimethylamine for synthesis Order number Packaging Quantity Price AC28119 Glass bottle 10 g 18.45 AC28119 Glass bottle 50 g 71.55 Product information Synonyms Dimethylaminoborane, Borane-dimethylamine-complex Hill Formula C2H10BN Chemical formula (CH3)2NHBH3 HS Code 2942 00 00 EC number 200-823-7 Molar mass 58.92g/mol CAS number 74-94-2 Chemical and physical data Ignition temperature 175C Solubility 128g/l (20C) Melting point 33-36C Molar mass 58.92g/mol Density 0.73g/cm3 (20C) Bulk density 300-350kg/m3 pH value 8-9 (10g/l, H2O, 20C) Vapor pressure <-5hPa (20C) Flash point 65C Safety information R Phrase R 24/25-36-51/53Toxic in contact with skin and if swallowed.Irritating to eyes.Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. S Phrase S 6-36/37-45-61Keep under argon.Wear suitable protective clothing and gloves.In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible).Avoid release to the environment. Refer to special instructions/ Safety data sheets. Categories of danger toxic, irritant, dangerous for the environment Hazard Symbol Toxic Dangerous for the environment RTECS IP9450000 Storage class 6.1A Combustible substances, toxic WGK WGK 2 water endangering Disposal 26Alkali and alkaline-earth metals should be taken up in an inert solvent and neutralized by adding 2-propanol (Cat. No. 100995) dropwise with stirring. Should the reaction be more violent than expected, conversion should be carried out with tert-butanol or octanol. Caution: This reaction produces hydrogen, which can form explosive mixtures; take necessary precautions. When the reaction has ceased, add water dropwise; neutralize; container D. In the case of alkali or alkaline-earth borohydrides, add methanol (Cat. No. 106008) with stirring; in the case of alkali or alkaline-earth amides and hydrides, organoaluminium and organotin hydrides, add 2-propanol (Cat. No. 100995) dropwise. The substances, which are usually in solid form, should be previously suspended in an ether! When the respective reaction has ceased, hydrolize with water; then neutralize. Container D or E. Lithium aluminium hydride must also be destroyed by slurrying in an ether. Under an inert gas and with thorough stirring, add dropwise a 1: 4 mixture of ethyl acetate (Cat. No. 822277) and the ether used to prepare the slurry. Always ensure thorough mixing (stirring)! Container A. Transport information Declaration (transport by sea) IMDG-Code UN 2926 FLAMMABLE SOLID, TOXIC, ORGANIC, N.O.S.(DIMETHYLAMINEBORANE), 4.1 (6.1), II Declaration (transport by air) IATA-DGR UN 2926 FLAMMABLE SOLID, TOXIC, ORGANIC, N.O.S.(DIMETHYLAMINEBORANE), 4.1 (6.1), II Toxicological data LD 50 oral LD50 rat 59 mg/kg LD 50 dermal LD50 rabbit 210 mg/kg Specifications Assay(iodometric) 97% Melting range - lower value 33C - upper value 36C Identity (IR) passes testFAQs of Boron hydride dimethylamine:
Q: What is the purity level of Boron hydride dimethylamine?
A: The purity level of Boron hydride dimethylamine is 99.99%.Q: What type of chemical is Boron hydride dimethylamine?
A: Boron hydride dimethylamine is categorized as an Industrial Lab Chemical.Q: What are the storage instructions for Boron hydride dimethylamine?
A: Boron hydride dimethylamine should be stored in a dry place.Q: What is the grade of Boron hydride dimethylamine?
A: The grade of Boron hydride dimethylamine is Industrial.Q: What is the primary application of Boron hydride dimethylamine?
A: Boron hydride dimethylamine is primarily used for laboratory chemical purposes.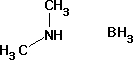

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Laboratory Chemicals Category
6-AMINOBENZOTHIAZOLE
Price 8065 INR
Minimum Order Quantity : 1 Gram
Grade : Analytical Grade
Type : Industrial Lab Chemicals
Purity(%) : 99%
Storage Instructions : Store in a cool, dry place
CADMIUM BROMIDE AR
Price 4657 INR / Gram
Minimum Order Quantity : 500 Grams, ,
Grade : Laboratory Grade
Type : University Lab Chemicals
Purity(%) : 99%
Storage Instructions : Store in a cool, dry place, keep tightly closed
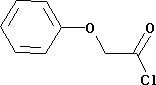
Phenoxyacetyl Chloride
Minimum Order Quantity : 500 Millimeters
Grade : Industrial
Type : Industrial Lab Chemicals
Purity(%) : 99.99
Storage Instructions : Dry Place
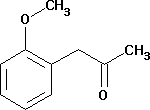
(2-Methoxyphenyl) acetone
Minimum Order Quantity : 500 Milliliters
Grade : Industrial
Type : Industrial Lab Chemicals
Purity(%) : 99.99
Storage Instructions : Dry Place
"Only deals in retail accepting orders upto 500ml only".
 |
ALPHA CHEMIKA
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese


 Send Inquiry
Send Inquiry